Life Enhancing Medications
As an emerging specialty pharmaceutical company, Pangea Pharmaceuticals, leverages its proven expertise to advance its portfolio of therapeutics and comprehensive support services, all designed to deliver meaningful outcomes for patients.
Learn More
Pangea Pharmaceuticals
Pangea has a strong commitment to patients by creating access to enhanced, safe, and more affordable medicines. In our effort to improve patient access to life-enhancing prescription medications, we offer a robust portfolio of branded and generic drugs for the pediatric health, pain management, neurological, and ophthalmic segments.
Biz Dev
We are constantly evaluating product opportunities to add to our commercial portfolio and late stage product development candidates across various therapeutic categories and dosage forms.
Our Focus
Our focus is to establish meaningful distribution relationships with hospitals, clinics, independent and specialty pharmacies, GPOs, and niche and speciality distributors.
Our Team
Decades of experience in the US pharmaceutical market provides an unparalleled experience to our partners and access to our global sourcing network of API and Contract Manufacturing partners.
Our Advantage
As a US-based specialty pharmaceutical company our streamlined structure promotes decisive action and a rapid go-to-market strategy for our products and partners.
89yrs
Combined Experience
With our combined experience, we bring a wealth of knowledge, expertise, and innovation to deliver exceptional results for our clients
6+
Pharmaceutical Products
Our pharmaceutical offerings are crafted with the highest standards of quality and safety, ensuring effective and reliable solutions for your health and well-being
12+
In Development
Our pharmaceutical products in development are designed to meet current and emerging patient needs as well as bridge gaps in the market
Core Strengths
Pangea's core strengths and our unwavering commitment to delivering high-quality specialty medications with precision and reliability
-
Life Enhancing Medicines
-
Leveraging Market Opportunities
-
Aquisitions & Licensing
-
Brand Development and Marketing
Our Products
Our pharmaceutical offerings are crafted with the highest standards of quality and safety, ensuring effective and reliable solutions for patient's health and well-being. Our listing of exclusive and future development products can be found below.
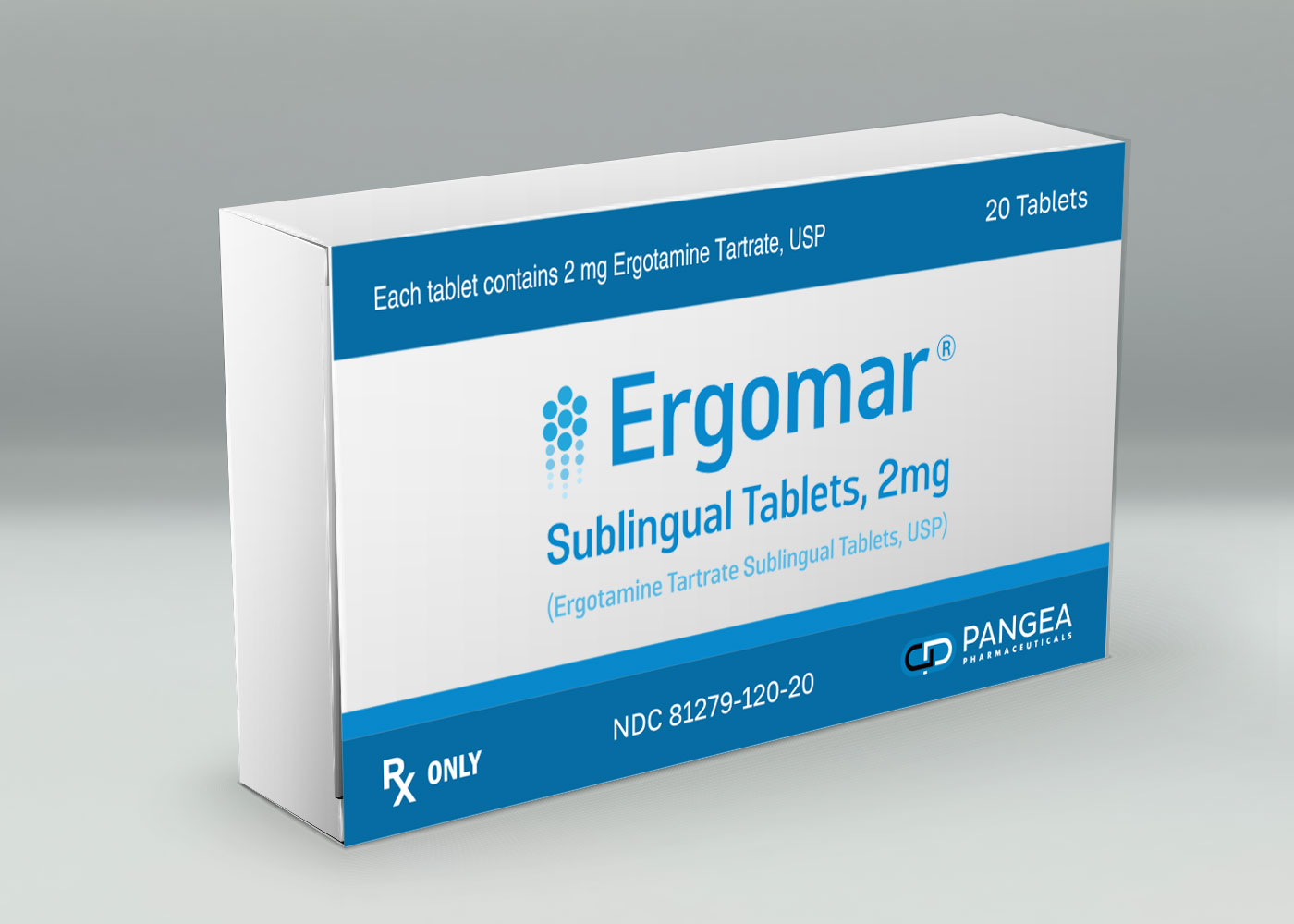
Ergomar
Migraine Relief
The only FDA Approved sublingulal treatment. Abort or prevent vascular headaches such as migraine, migraine variants, or so called "histaminic cephalagia".
Bucapsol
Anxiety Relief
Buspirone Hydrochloride capsules providing anxiety relief that is non-sedating, non-addictive, and safe for long-term use.
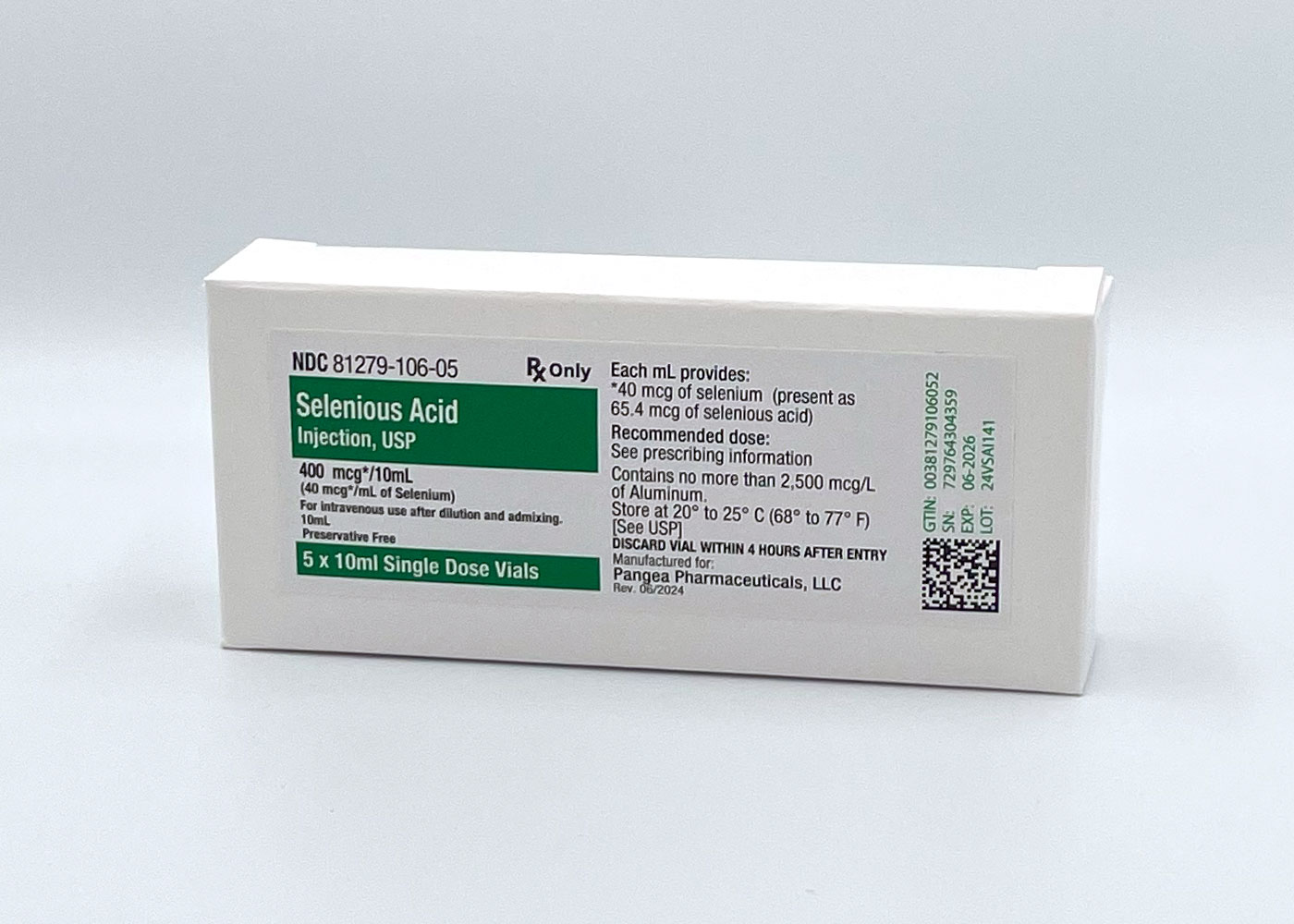
Selenious Acid
IV Supplement
Selenious acid is a selenium supplement used to treat low selenium levels. It's added to total parenteral nutrition (TPN) and infused through the veins.
Our Pipeline
Our determination to deliver meaningful outcomes for patients motivates us to pursue agressive development. Our product pipeline includes new molecular entities as well as existing products with potential new indications or different formulations.
| PID | Category | Development | Clinical | Reg Review | FDA Approval | Est Launch |
|---|---|---|---|---|---|---|
|
P-010
|
Antihistamine
|
|
Q3 2026 |
|||
|
P-042
|
Ophthalmic Mydriatic/Cycloplegic
|
|
Q4 2026 |
|||
|
P-041
|
Ophthalmic NSAID
|
|
Q3 2026 |
|||
|
P-040
|
Calcium Channel Blocker
|
|
Q1 2026 |
|||
|
P-039
|
NASID
|
|
Q1 2026 |
|||
|
P-038
|
Anti-inflammatory
|
|
Q3 2026 |
|||
|
P-006
|
Muscle Relaxant
|
|
Q4 2027 |
|||
|
P-003
|
NASID
|
|
Q1 2027 |
|||
|
P-014
|
Oxidation-Reduction Agent
|
|
Q3 2026 |
|||
|
P-004
|
NASID
|
|
Q3 2026 |
|||
|
P-037
|
NASID
|
|
Q2 2026 |
|||
|
P-001
|
Antihistamine
|
|
Q2 2026 |
|||
|
P-031
|
Anticonvulsant
|
|
Q2 2026 |
|||
|
P-013
|
Antibacterial Agent
|
|
Q3 2026 |
|||
|
P-002
|
NSAID
|
|
Q4 2026 |
|||
|
P-012
|
Anticholinergic
|
|
Q1 2026 |
|||
|
P-008
|
Gallstone Dissolution Agent
|
|
Q1 2026 |
|||
|
P-007
|
Glucocorticosteroid
|
|
Q1 2026 |
|||
|
P-009
|
Anticholinergic
|
|
Q1 2026 |
|||
|
P-029
|
Urinary Anti-infective
|
|
Q1 2026 |
|||
| Development | Clinical | Reg Review | FDA Approval |
|---|---|---|---|
|
P-010
Antihistamine
Q3 2026
|
|||
|
P-042
Ophthalmic Mydriatic/Cycloplegic
Q4 2026
|
|||
|
P-041
Ophthalmic NSAID
Q3 2026
|
|||
|
P-040
Calcium Channel Blocker
Q1 2026
|
|||
|
P-039
NASID
Q1 2026
|
|||
|
P-038
Anti-inflammatory
Q3 2026
|
|||
|
P-006
Muscle Relaxant
Q4 2027
|
|||
|
P-003
NASID
Q1 2027
|
|||
|
P-014
Oxidation-Reduction Agent
Q3 2026
|
|||
|
P-004
NASID
Q3 2026
|
|||
|
P-037
NASID
Q2 2026
|
|||
|
P-001
Antihistamine
Q2 2026
|
|||
|
P-031
Anticonvulsant
Q2 2026
|
|||
|
P-013
Antibacterial Agent
Q3 2026
|
|||
|
P-002
NSAID
Q4 2026
|
|||
|
P-012
Anticholinergic
Q1 2026
|
|||
|
P-008
Gallstone Dissolution Agent
Q1 2026
|
|||
|
P-007
Glucocorticosteroid
Q1 2026
|
|||
|
P-009
Anticholinergic
Q1 2026
|
|||
|
P-029
Urinary Anti-infective
Q1 2026
|
|||

Pharmaceutical Partnerships
Pangea boasts strong distribution relationships with US retail chains, wholesalers, distributors, managed care organizations, mail order pharmacies, and Group Purchasing Organizations (GPOs).
Our robust commercial infrastructure, extensive experience, diverse distribution network, and our ability to execute efficient launches facilitate strategic business alliances and focused collaborations with other specialty pharmaceutical organizations. Please direct partnership inquiries to our commerical team.